Research
I. Characterization of molecular mechanisms of EBOV entry and virion formation: toward the development of therapeutics for EBOV disease
Ebolavirus (EBOV), a member of the family Filoviridae, is an enveloped, single-stranded, negative-sense RNA virus that causes severe hemorrhagic fever with a high mortality rate, known as EBOV disease (EVD), in humans and nonhuman primates. Currently, no specific therapeutics has been approved for treatment and prevention of EVD. Although EBOV entry and virion formation processes would be a desirable target for anti-therapeutics, the precise mechanism of this process remains poorly understood. This lack in understanding derives in part from the requirement for BSL-4 containment for working with wild-type EBOV. Because of the likelihood of future outbreaks and generation of mutant viruses, the development of a variety of EBOV therapeutics is urgent. Our goal is to elucidate the mechanism underlying virus entry and virion formation processes with a focus on host membrane traffic dynamics by taking advantage of the BSL-4 facility, which will be launched at Nagasaki University.
1. Characterization of the mechanism by which EBOV is internalized into host cells
We established a real-time visualization system for the internalization of EBOV particles and demonstrated that EBOV enters the host cells via macropinocytosis in a viral glycoprotein (GP)-dependent manner (Nanbo et al. PLoS Pathog, 2010). Previous reports demonstrate that phosphatidylserine (PS) receptors appear to contribute to EBOV entry, indicating PS exposed on viral envelop and GP synergistically contribute to the entry process. EBOV virions bud from the plasma membrane. In the normal cells, PS typically distributes in the inner leaflet of the plasma membrane, suggesting that PS is exposed on its outer leaflet at sites of egress. We have demonstrated host scramblase, Xkr8 involves in the externalization of PS on the virion envelop (Nanbo et al. PLoS Pathog, 2016). We are currently developing the therapeutics that specifically target the entry and virion formation process in multiple ways (Isono et al, Antivir research, 2020, Furuyama et al. Sci Rep, 2016, Kuroda et al. J Virol, 2015), which should lead to the significant contribution for prevention of EBOV infection in the future.
2. Characterization of the mechanism of EBOV virion formation
Although the viral replication process is also defined as an important target for therapeutics, the mechanism of viral particles formation remains unclear. We are characterizing the spatio-temporal distribution of EBOV proteins and RNAs by using a recombinant EBOV (Nanbo et al. Sci Rep, 2013) to clarify the molecular mechanism of assembly of viral particles. In addition, we are elucidating the role of host membrane dynamics in the process of virion formation (Nanbo et al. J Infect Dis, 2018) (Nanto et al, in submission),
II. Characterization of molecular mechanism of development of Epstein-Barr virus-associated epithelium tumors
Epstein-Barr virus (EBV), a ubiquitous human γ-herpesvirus, establishes a persistent latent infection in B lymphocytes and epithelial cells in more than 90% of adults worldwide. Although this virus contributes causally to lymphomas and epithelial malignancies such as Burkitt’s lymphoma, gastric carcinoma, and nasopharyngeal carcinoma, the molecular mechanism by which EBV cause these tumors remains fully elucidated.
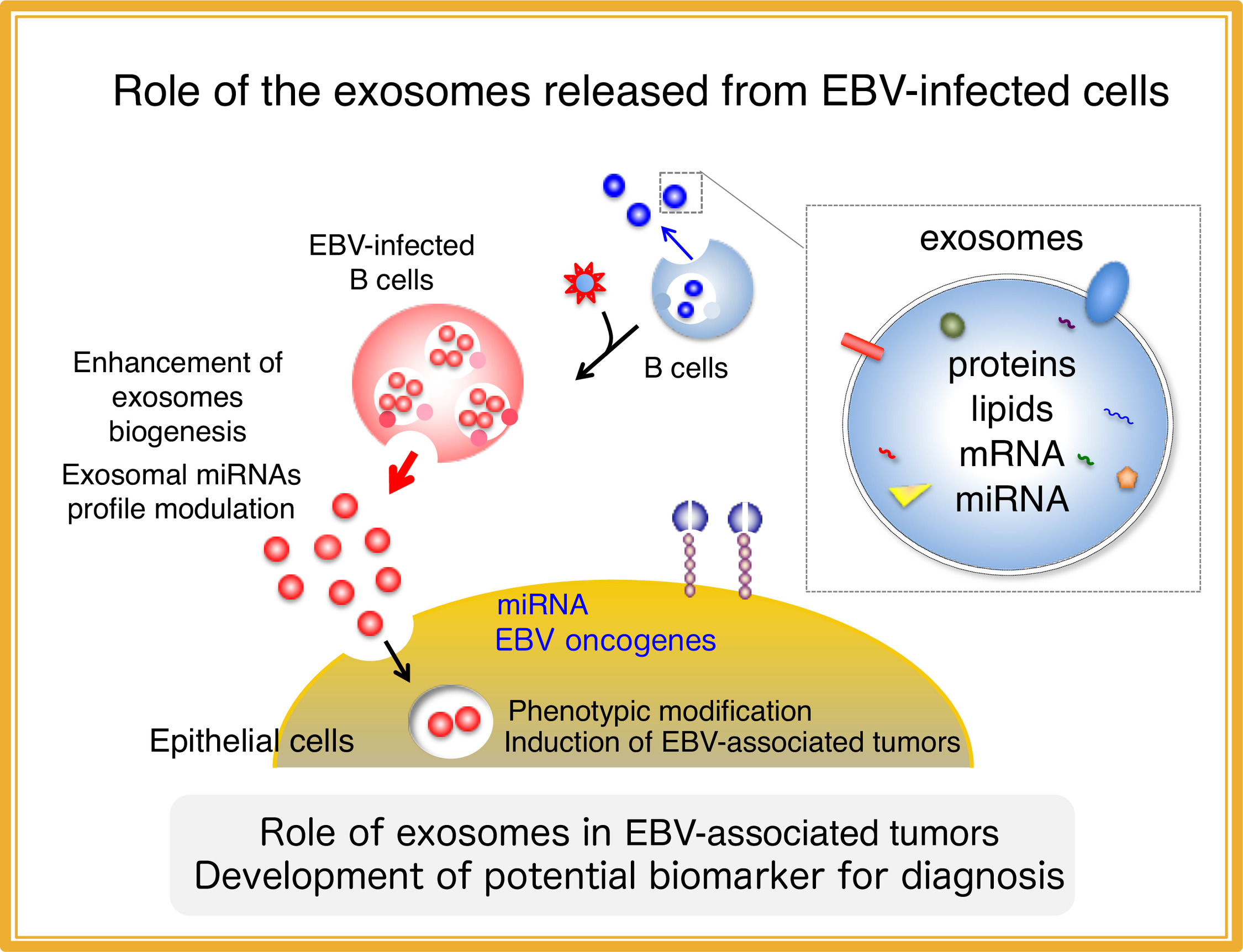
1. Role of exosomes released from EBV-infected cells
To update the understanding of the mechanisms for development of EBV-associated epithelial tumors, we are elucidating the physiological significance of extracellular microvesicles, exosomes released from EBV-infected cells in tumor development (Nanbo et al. J Virol, 2013). Moreover, we try to identify host and viral factors that are specifically and abundantly incorporated in exosomes (Nanbo et al. Cancers, 2018), which shall lead to the development of potential biomarkers for EBV-associated tumors that contribute to the diagnosis of these tumors.
2. Molecular mechanism of cell-to-cell contact-mediated EBV transmission in epithelial cells
Several lines of evidence indicate that infection of EBV into epithelial cells is significantly enhanced by co-culturing with latently EBV-infected B cells, indicating that infection of epithelial cells by EBV is predominantly mediated by cell-to-cell contact. We are investigating the molecular mechanisms by which EBV exploits cell contact-induced cell signaling pathways (Nanbo et al. J Virol, 2012), intracellular membrane dynamics (Nanbo et al. J Gen Virol, 2016) and production of growth factors (Nanbo et al. Front Microbiol, 2018) to establish the efficient viral transmission.
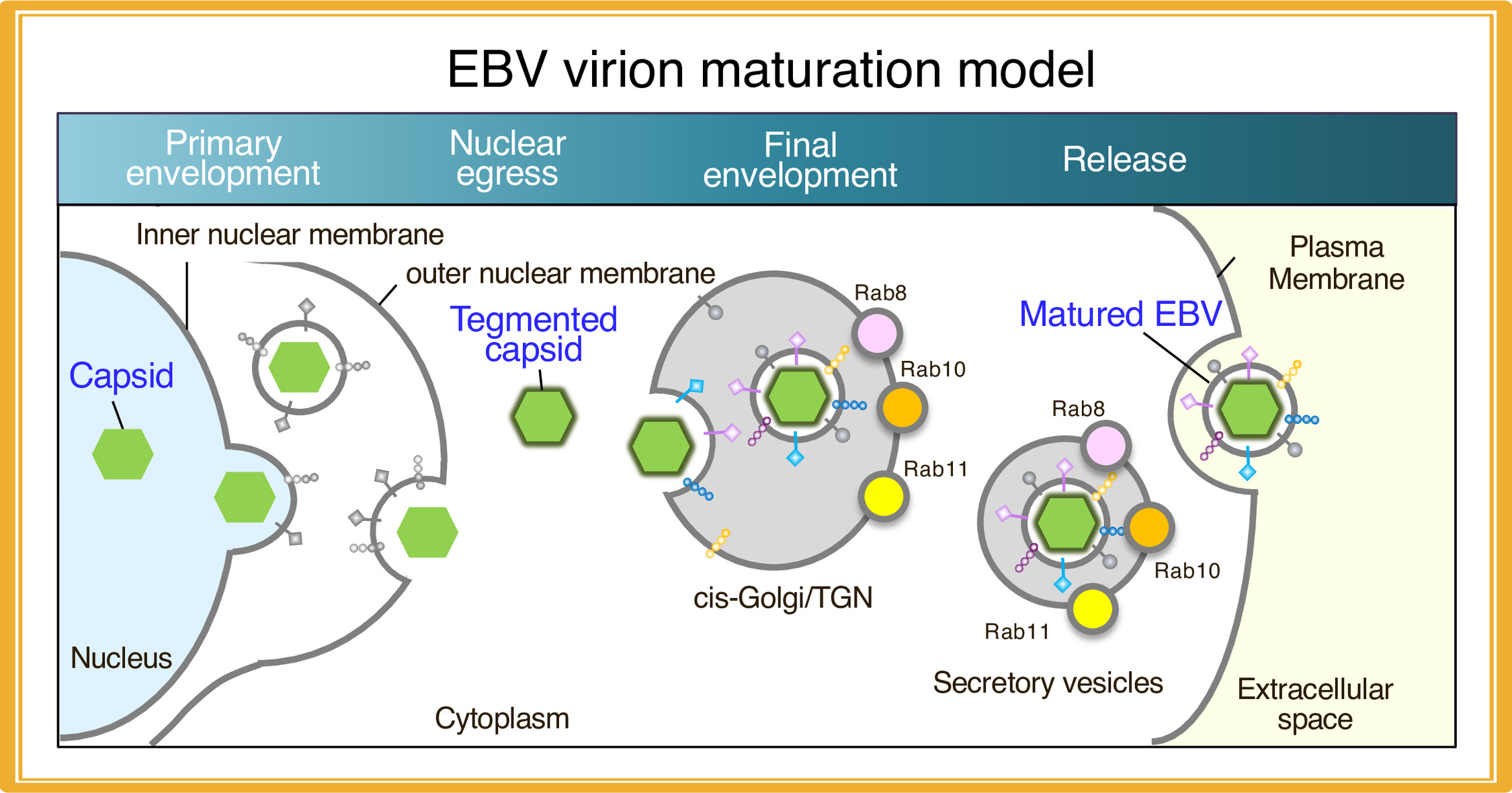
3. Characterization of maturation of EBV virions
It has been shown that herpesvirus subfamilies share a novel mechanism for maturation and egress of virions through several budding and fusion events during replication, which prevents disruption of cellular membranes. We are characterizing the sites for the final envelopment of EBV in Burkitt’s lymphoma cell lines induced into the lytic cycle. EBV acquires its final envelope in intracellular compartments containing markers of Golgi apparatus (Nanbo et al. Front Microbiol, 2018) and subsequently are released to the extracellular milieu via the secretory pathway (Nanbo et al. Microorganisms, 2020), providing new insights into maturation of EBV virions.